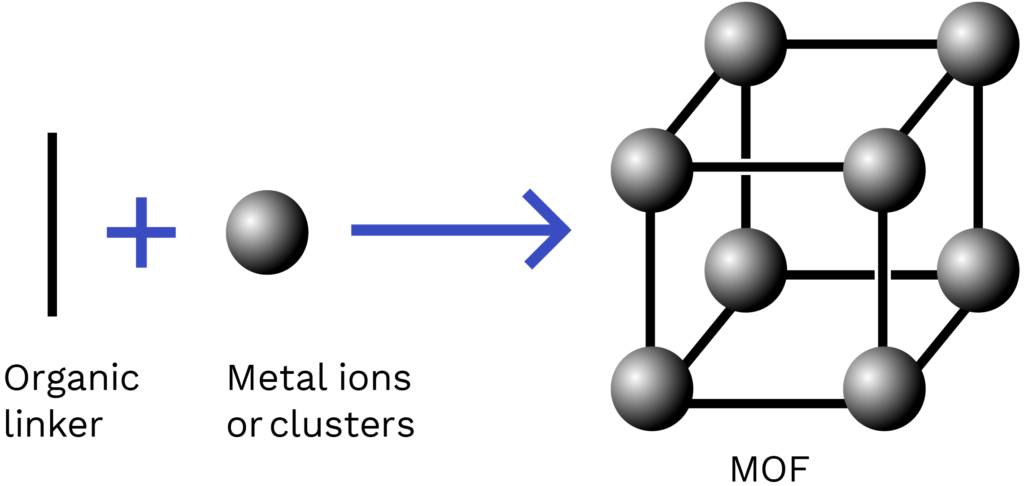
Metal-organic frameworks (MOFs) are constructed from metal ‘nodes’ and organic molecules known as ‘linkers’. The organic linkers coordinate to the metal clusters to form 1D, 2D and 3D structures.
These 3D frameworks are porous and can be filled with ‘guest’ molecules, just like a sponge absorbs water.
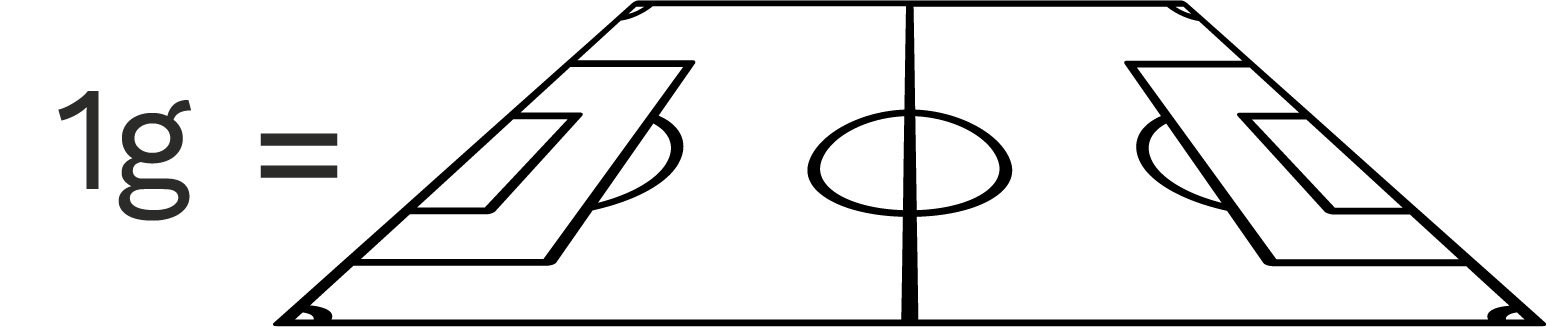
Millions of tiny holes in MOFs create huge surfaces. 1 gram of MOF has nearly the same surface area as a football pitch!
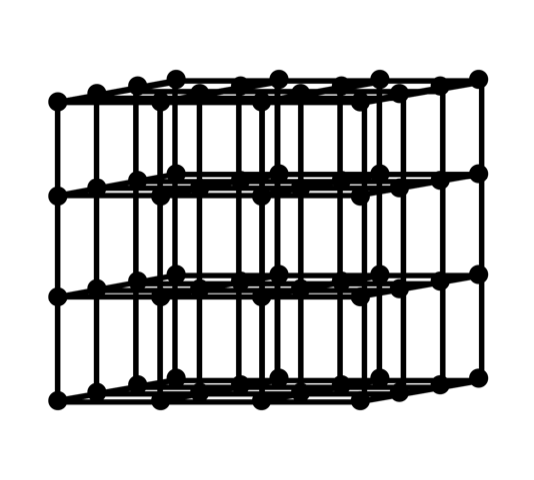
MOFs make miniature cages from metal nodes and organic structs. Cages can be assembled to make strong and porous crystalline materials.

We can change the nodes and the struts to create many possible MOFs with different abilities. Over 80,000 MOFs have been made so far.
MOFs might be valuable in everything from purification and separation, through catalysis and carbon capture, to clean energy, drug delivery systems and detection.
MOFs have large internal surface areas and can be assembled from a huge choice of different organic linkers and metals. This means their properties can be tuned for different applications.
These features make them ideal for the removal of toxic pollutants, as they can be designed to target and specifically remove gases like sulphur dioxide and nitrogen dioxide from exhaust gases and other pollution sources.
Once the toxic gases have been captured by the MOF they can be removed to make new and valuable chemicals including sulphuric and nitric acids.
After the MOF has been cleaned it can be reused again and again to capture more pollutants.
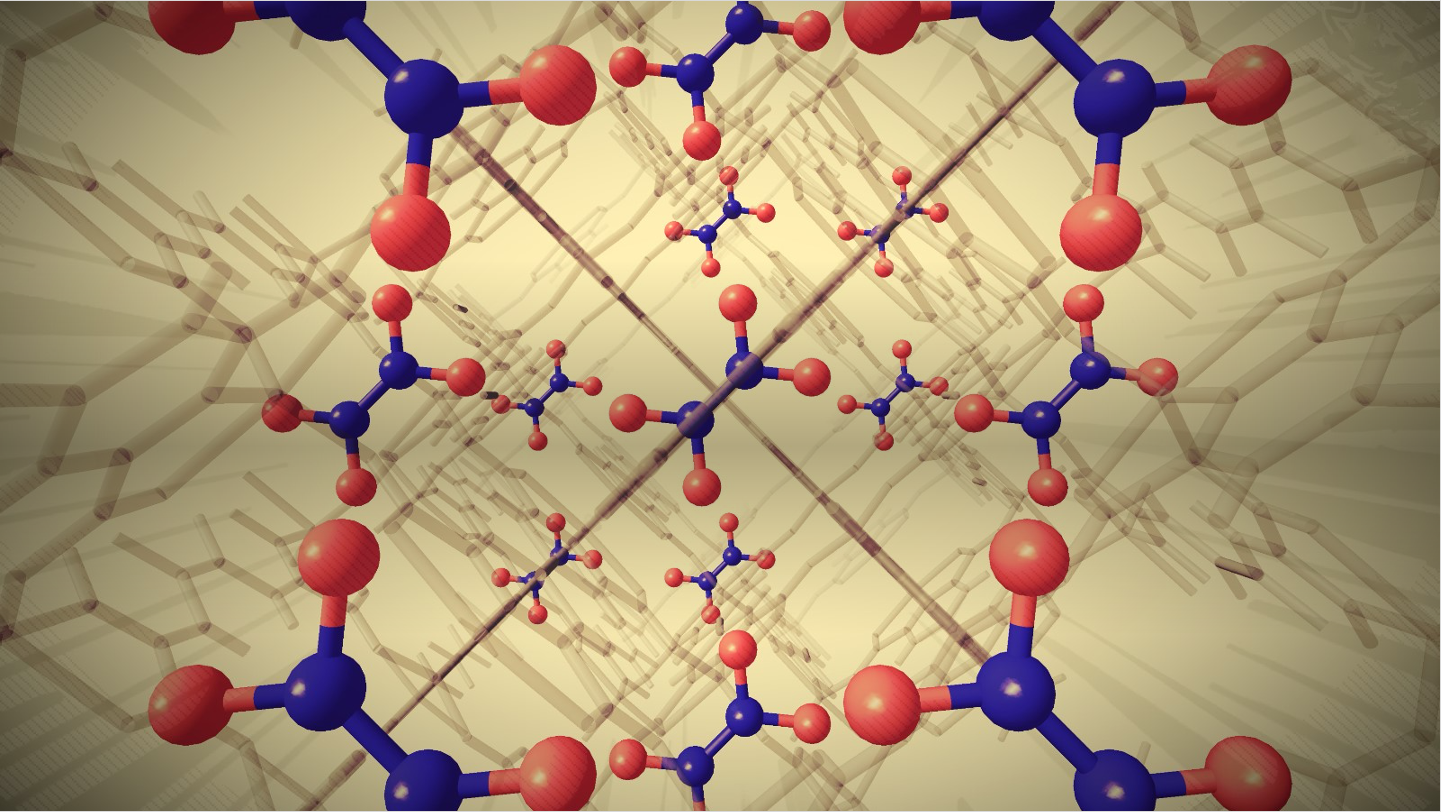
Artistic image showing how nitrogen dioxide molecules can be captured by a particular MOF called MFM-520. This process is called dimerization. The structure has been visualised using a special technique called ‘single crystal X-ray diffraction’
Nitrogen dioxide and sulphur dioxide are present in tiny quantities but are still harmful to our health. In untreated exhaust gas there is just 1 nitrogen dioxide or sulphur dioxide molecule in every 1,000 to 10,000 nitrogen and oxygen molecules. Both oxide gases are acidic and corrosive.
Different molecules interact with each other very differently. We make materials that can specifically interact with nitrogen dioxide and/or sulphur dioxide, but not with nitrogen or oxygen.
When air contaminated with Nitrogen dioxide passes through our material, only nitrogen dioxide is retained inside, and nitrogen and oxygen can pass through freely. In other words, the air is cleaned of pollutants!
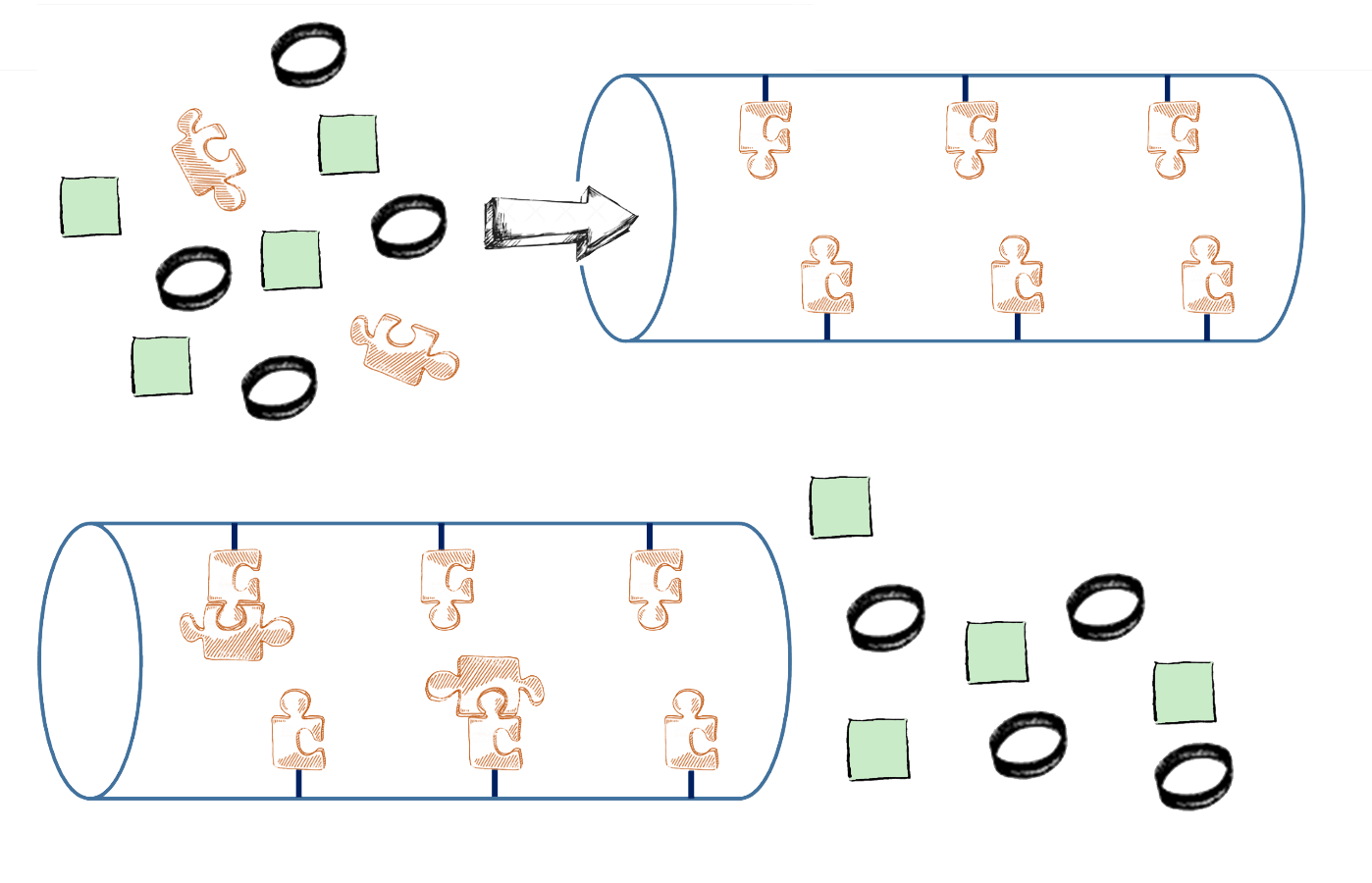
Graphic demonstrating how specific gases can be targeted and selectively trapped. The mixed gas is represented by 3 different shapes which travel through the channels inside the MOF. Only the complementary puzzle pieces stick inside the MOF, whilst the other shapes pass through the other side.
You can explore more cutting-edge science with the Royal Society at royalsociety.org/summer